By Erin Maxwell || News Editor

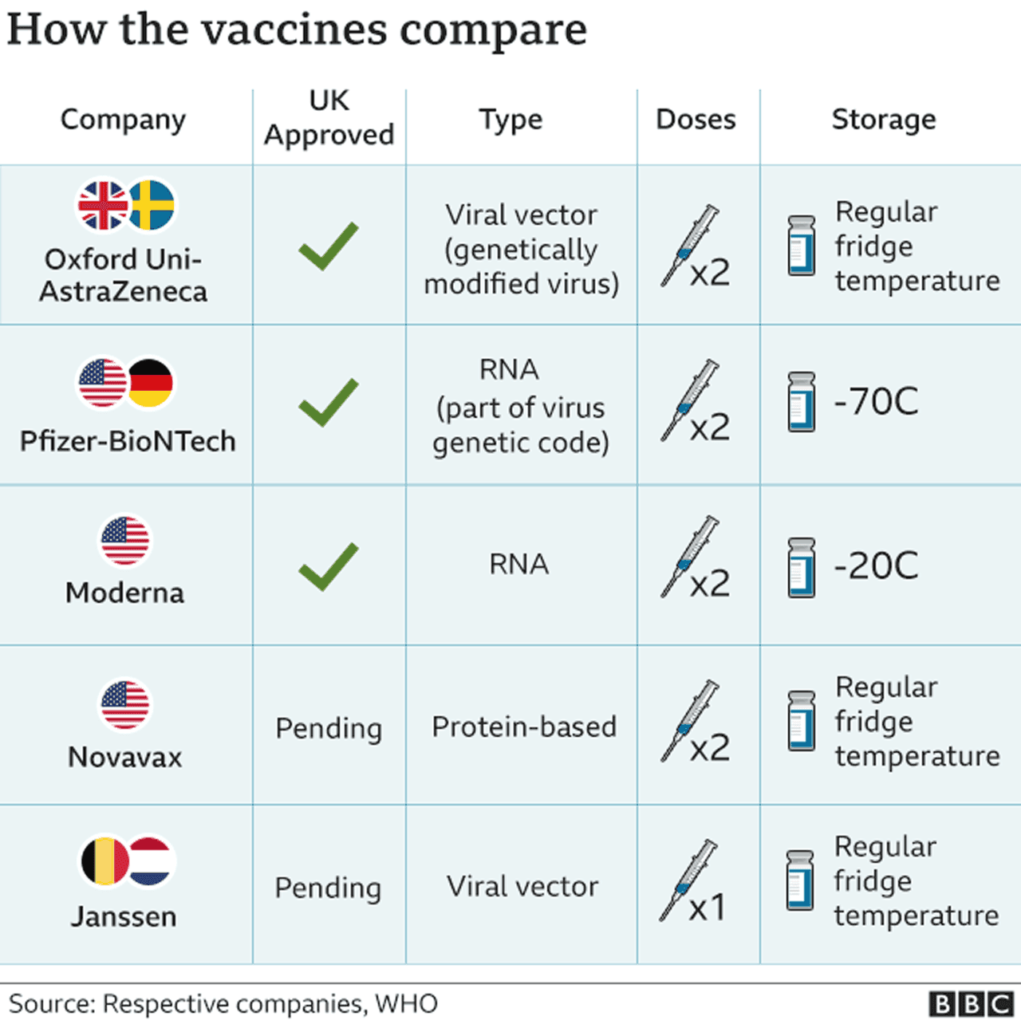
As the vaccine rollout goes into hyperspeed to combat rising case numbers, a massive roadblock in the introduction of a third inoculation formed last week, with the announcement of unexplained blood clot phenomena in Johnson & Johnson recipients. On April 13th, the CDC and FDA released a joint statement recommending a nationwide pause on the utilization of the Johnson & Johnson vaccination, as the CDC’s Advisory Committee on Immunization (ACIP) undergoes an investigation into six instances of a rare clotting condition referred to as “cerebral venous sinus thrombosis”, or CVST.
As of April 12, more than 6.8 million doses of the J&J shot had been administered nationwide, although some production delays had kept rollout at a pithy 3% of total vaccinations. Among those vaccinated, six women, all between the ages of 18 to 48, were diagnosed with CVST, an extremely rare form of a blood clot occurring in the brain’s arteries. All six of these women also presented an extremely low platelet count, making the standard treatment of the blood thinner heparin dangerous. One of these cases proved fatal, causing the passing of a Virginia woman, with another woman currently in critical condition in Nebraska.
Although these events are concerning, top health officials have continually stressed the rarity of these occurrences, which make up “six out of the 6.85 million doses, which is less than 1 in a million,” according to Dr. Anthony Fauci, who spoke in a White House briefing this Tuesday (ABC News). In comparison to these minimal statistics, the likelihood of developing a blood clot after hospitalization for COVID-19, the target of this vaccine, could be as high as 31%, or 1 in 6 patients. “Remember,” said Dr. Kelly Moore, deputy director of the Immunization Action Coalition, “The disease this vaccine prevents is a major killer” (USA Today).
Across the ocean, the European Union is facing a similar foe in the AstraZeneca vaccine, whose 34 million doses have been linked to 220 blood clotting incidents as of April 4th. A similar temporary pause in its distribution was levied earlier this month, with the European Medicines Agency concluding that this disorder was an extremely rare side effect that did not outweigh the benefits of the inoculation. Many researchers are attributing this common effect of the AstraZeneca and J&J shot to their shared adenovirus technology, which carries DNA into human cells to generate immunity. This distinguishes these two from the mRNA technology of the Pfizer and Moderna vaccines, which have not produced any similar side effects.
300,000 to 600,000 American citizens suffer from blood clots each year, but this particular diagnosis “just doesn’t happen in healthy people out of the blue, and that’s what’s happening,” said Dr. Hanny Al-Samakri of Massachusetts General Hospital (USA Today). Many hypotheses have been submitted to trace a causal factor, the most notable of which coming from Dr. Theodore Warkentin in the New England Journal of Medicine. Warkentin’s theory suggests that vaccines trigger what he calls a “vaccine-induced immune thrombotic thrombocytopenia”, or VITT. This effect involves the immune system producing antibodies, which in turn activate platelets in the bloodstream that clump into clots. The true causation is still unknown, as are any risk factors that may contribute to an individual’s propensity to develop a clot.
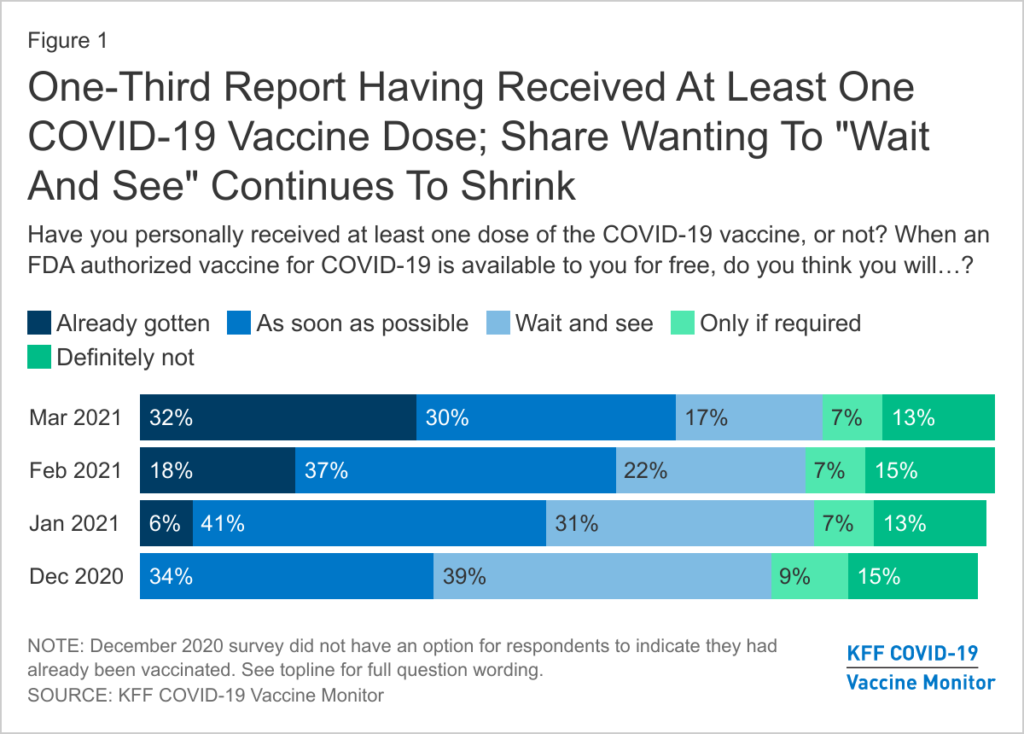
The U.S., South Africa, and the EU have all temporarily paused J&J’s rollout, but growing concerns around vaccine hesitancy have prompted continuous disclaimers from top health officials. Pfizer and Moderna doses have produced enough dosage to meet Biden’s “end of May” goal, but the inoculation effort relies not only on logistics but on public faith. According to the Kaiser Family Foundation’s most current COVID-19 Vaccine Monitor, a persistent pocket of Americans either responded that they would “definitely not” receive the vaccine, or would “wait and see” (KFF). Among these two respondent categories, 7 out of 10 reported side effects as their main concern. Last week, a huge amount of vaccination appointments were canceled in light of the J&J news, complicating efforts during a nationwide surge in cases. In order to achieve the goal of herd immunity, “we have to do everything possible to avoid a collapse in confidence in vaccination overall,” insists New York City Councilman Mark Levine, echoing the concerns of his colleagues (LA Times).
Findings of the joint CDC and FDA investigation are expected soon, an undertaking they claim is a result of “an abundance of caution” (FDA). Until then, Pfizer and Moderna doses continue to steadily increase the population of vaccinated individuals nationwide.
Editor’s Note: Lancaster County is currently administering the Pfizer and Moderna vaccinations to all eligible residents. The College Reporter strongly supports all students and residents in Lancaster to visit vaccinatelancaster.org for information, and to register for their vaccination as soon as possible.
Sophomore Erin Maxwell is the News Editor for The College Reporter. Her email address is: emaxwell@fandm.edu.
